Overview
 We are broadly interested in drugs that may be used for treating depression. We want to know how therapeutic behavioral effects are produced by a drug’s action on synapses and neurons in the brain. Many of our experiments involve optical imaging and electrophysiology in awake mice. By gaining biological insights and engineering new tools, our long-term goal is to discover effective strategies for treating depression. Current projects in the lab focus on ketamine and psychedelics such as psilocybin and 5-MeO-DMT (Kwan et al., Nat Neurosci 2022; Kelmendi et al., Curr Biol 2022). We have made progress in three areas.
We are broadly interested in drugs that may be used for treating depression. We want to know how therapeutic behavioral effects are produced by a drug’s action on synapses and neurons in the brain. Many of our experiments involve optical imaging and electrophysiology in awake mice. By gaining biological insights and engineering new tools, our long-term goal is to discover effective strategies for treating depression. Current projects in the lab focus on ketamine and psychedelics such as psilocybin and 5-MeO-DMT (Kwan et al., Nat Neurosci 2022; Kelmendi et al., Curr Biol 2022). We have made progress in three areas.
Drug action on synapses and dendrites
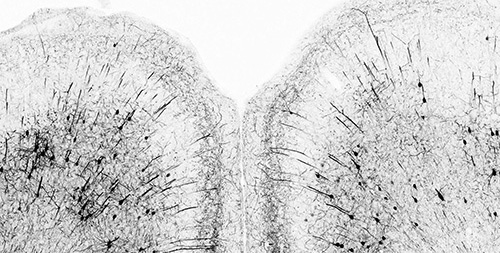 Depression is associated with a loss of synapses in the prefrontal cortex. There is growing evidence that antidepressants may counteract the deficit by enhancing synaptic plasticity. Our lab initially used two-photon microscopy to track how ketamine induces the rapid remodeling of neuronal connections in a living brain (Phoumthipphavong et al., eNeuro 2016). Subsequent studies showed drug-evoked structural rewiring that persists for many weeks after a single dose of psilocybin (Shao et al., Neuron 2021) or 5-MeO-DMT (Jefferson et al., NPP 2023). We are keen to delineate the molecular and cellular mechanisms that underlie the enduring structural plasticity (Liao et al., Nat Rev Neurosci 2025).
Depression is associated with a loss of synapses in the prefrontal cortex. There is growing evidence that antidepressants may counteract the deficit by enhancing synaptic plasticity. Our lab initially used two-photon microscopy to track how ketamine induces the rapid remodeling of neuronal connections in a living brain (Phoumthipphavong et al., eNeuro 2016). Subsequent studies showed drug-evoked structural rewiring that persists for many weeks after a single dose of psilocybin (Shao et al., Neuron 2021) or 5-MeO-DMT (Jefferson et al., NPP 2023). We are keen to delineate the molecular and cellular mechanisms that underlie the enduring structural plasticity (Liao et al., Nat Rev Neurosci 2025).
Drug action on neural circuit dynamics
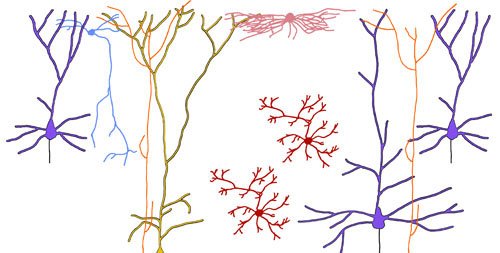 Behavior is generated by neural activity, which is orchestrated by excitatory and inhibitory neurons in the brain. However, we have a limited understanding of how antidepressants alter the firing patterns of various cell types. Our results uncovered the role of dendrite-targeting GABAergic neurons in elevating synaptic calcium signaling after ketamine administration (Ali et al., Nat Commun 2020). We found that a major subtype of pyramidal cells in the frontal cortex is essential for the long-lasting action of psilocybin (Shao et al., bioRxiv). We want to understand how the cortical microcircuit alters its firing dynamics in response to ketamine and psychedelics (Savalia et al., TINS 2021).
Behavior is generated by neural activity, which is orchestrated by excitatory and inhibitory neurons in the brain. However, we have a limited understanding of how antidepressants alter the firing patterns of various cell types. Our results uncovered the role of dendrite-targeting GABAergic neurons in elevating synaptic calcium signaling after ketamine administration (Ali et al., Nat Commun 2020). We found that a major subtype of pyramidal cells in the frontal cortex is essential for the long-lasting action of psilocybin (Shao et al., bioRxiv). We want to understand how the cortical microcircuit alters its firing dynamics in response to ketamine and psychedelics (Savalia et al., TINS 2021).
Novel screening methods for drug discovery
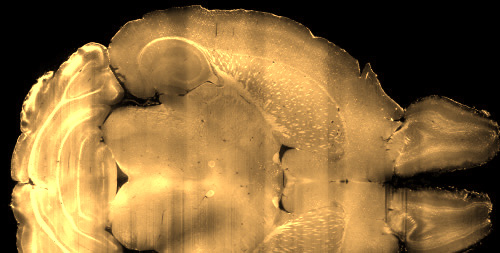 A challenge in drug development lies in the screen of hundreds of compounds to identify a lead candidate to advance to clinical trials. We believe assays that quantify drug action in native brain tissues can be a powerful approach. We created methods based on light sheet fluorescence microscopy, mapping the brain-wide expression of plasticity-related gene evoked by ketamine and psilocybin (Davoudian et al., ACS Chem Neurosci 2023). We developed a machine learning pipeline to use the gene expression maps to classify psychedelics and related psychoactive drugs (Aboharb et al., Nat Commun 2025). Most recently, we explored the impact of ketamine and psilocybin on transcriptomics in cortical cell types using single-cell sequencing techniques (Liao et al., bioRxiv).
A challenge in drug development lies in the screen of hundreds of compounds to identify a lead candidate to advance to clinical trials. We believe assays that quantify drug action in native brain tissues can be a powerful approach. We created methods based on light sheet fluorescence microscopy, mapping the brain-wide expression of plasticity-related gene evoked by ketamine and psilocybin (Davoudian et al., ACS Chem Neurosci 2023). We developed a machine learning pipeline to use the gene expression maps to classify psychedelics and related psychoactive drugs (Aboharb et al., Nat Commun 2025). Most recently, we explored the impact of ketamine and psilocybin on transcriptomics in cortical cell types using single-cell sequencing techniques (Liao et al., bioRxiv).
Funding
We are grateful for current and past support from NIMH, NINDS, NIA, Simons Foundation Autism Research Initiative, Brain & Behavior Research Foundation, One Mind, Epilepsy Foundation, Yale Program for Psychedelic Science, Yale CTNA, and Inscopix DECODE Program.
We appreciate fellowship support for trainees from NIMH, NIDA, NSF, Source Research Foundation, Brain & Behavior Research Foundation, Philippe Foundation, Alzheimer’s Association, and Alzheimer’s Drug Discovery Foundation.
